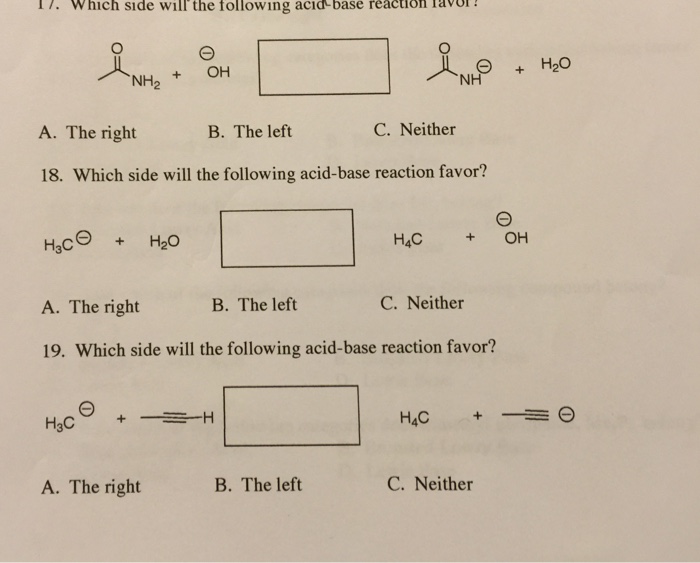
Which Side Will the Following Acid Base Reaction Favor
So we need to know the pKa of the acid on the left. For the following acid-base reaction predict which side the equilibrium is favored.
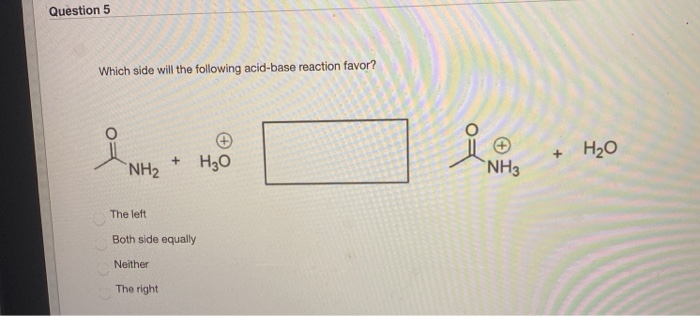
Solved Question 5 Which Side Will The Following Acid Base Chegg Com
Here C l X is the conjugate base of HCl which is a strong acid.
. Phenol acts as an acid and ethoxide ion act as the base. Key things to remember for acid-base reactions. The conjugate acid of water on the product side In base ionization.
Which side will the following acid-base reaction favor. In which direction will an acid-base reaction go. HCO3 H2PO4 H2CO3 HPO42 Enter your answers as either acid base conjugate acid or conjugate base.
Which side will the following acid-base reaction favor. Both the base and the conjugate base have the negative charge on a carbon atom. Favor left side C.
Click again to see term. For the following acid-base reaction predict which side the equilibrium is favored. For the following acid-base reaction predict which side of the equilibrium if favored.
This means that C l X is a really weak base meaning that it is pretty bad at pulling protons from molecules. Which of the following indicated Hs is the most acidic. Explain the reasoning for your answer.
Tap card to see definition. Acid Base Equilibrium Organic Chemistry Part 1 which side of an acid base reaction is favored HOW TO PREDICT THE EQUILIBRIUM DIRECT. B Favor Right Side.
The reason for this is that like any chemical reaction or a process the acid-base reactions go towards a lower energy state. Phenol is stable but ethoxide ion is not because there is no resonance stabilization in the ion. Click card to see definition.
The negative charge on the conjugate base is stabilized via resonance resulting in a more stable base. Because acids and bases are paired as conjugates the stronger acid is on the same side as the stronger base so the reaction that is energetically favored is the one where strongerstronger weakerweaker. C Favor Left Side.
Now lets look at the reverse reaction which is. Acid Base Equilibrium Organic Chemistry Part 3 which side of an acid base reaction is favored HOW TO PREDICT THE EQUILIBRIUM DIRECT. N a X C l X H X 2 O N a O H H C l.
For the following acid-base reaction predict which side of the equilibrium is favored. Any Acid-Base reaction favors the formation of a weaker acid and a weaker base. The stronger the acid the lower the pKa value.
For the following acid-base reaction predict which side of the equilibrium is favored. Up to 256 cash back Get the detailed answer. To the right 4.
A strong acid or a base means that they have a lot of energy and are very reactive while weaker acids and bases have lower energy. Amount concentration of material that ends up on the right divided by the amount of material on the left denoted by Keq. Which side will the following acid-base reaction favor.
While in the right side where phenoxide ion is stable as an ion due to resonance stabilization with the aryl ring and also making is a more stable weaker base. So we already know that acetic acid is the acid on the left side here and acetic acid has a pKa this proton right here has a pKa of approximately five. 33 Use p K values to compare acidity and basicity and to predict.
Put a circle around the acid-base reactions below that should favor the products. For the following acid-base reaction predict which side the equilibrium is favored. It will proceed away from the stronger acid and base toward the weaker acid and base the reaction will favor the side of the weaker acid and base.
To the left c. - V HO HO H2O HO H-CC-CH2 H2O O CC-CH3 NH2 NH3 O CEC-CH3 H-CC-CH3 I н H-CCCHE - H-CECCH3 B CEC-CH3 H I H. Favors the right side.
Which is the conjugate acid in the following reaction. So if we know the pKa values for the two acids in our reaction we can figure out the equilibrium constant for that reaction. Water H2O act as the acid on the reactant side and OH-is the conjugate base of water on the product side Dont include water in acid and base ionization equilibrium constant equations because it is a liquid and the reaction takes place in the aqueous phase which is.
Equilibrium favors the side of the weaker acid. For the following equation how many hydrogen atoms are added or lost. For the following acid-base reaction predict which side of the equilibrium is favored.
Identify the acid and base on the reactant side and the conjugate acid and conjugate base on the product side of the following reaction. Favor right side favor left side neither OneClass. Favor right side B.
In the following reaction how many Hs do you add or lose. O NH 2 O NH 3 H 2 O H 3 O A.
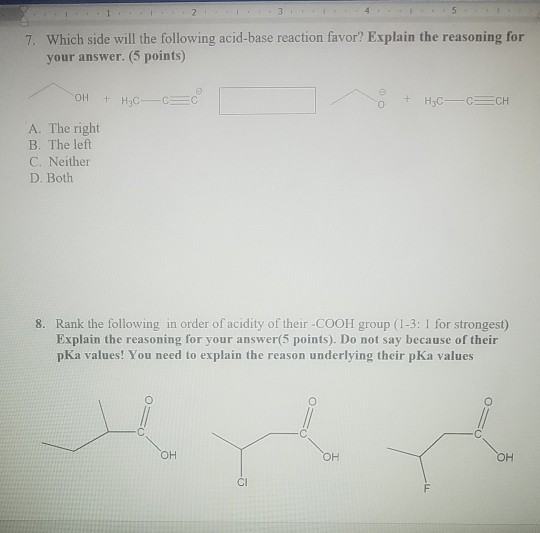
Solved 7 Which Side Will The Following Acid Base Reaction Chegg Com

Acid Base Equilibrium Organic Chemistry Video Clutch Prep
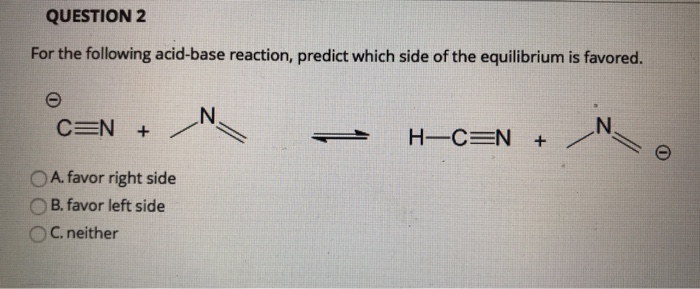
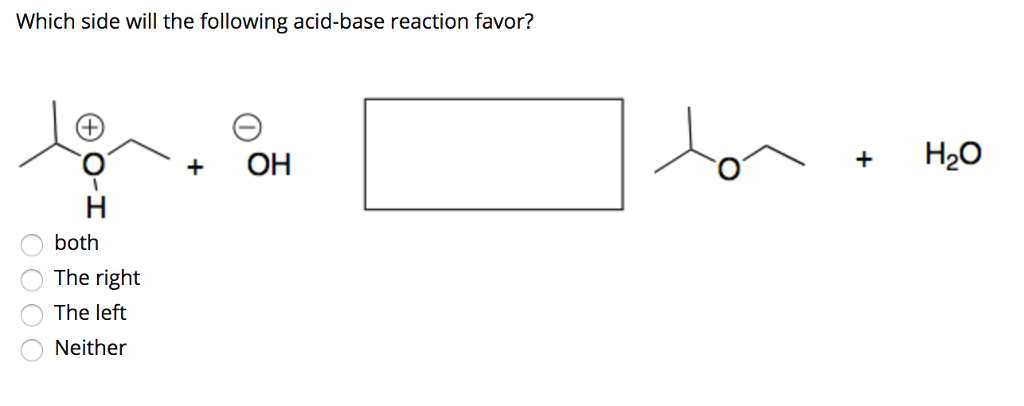
Solved Question 2 For The Following Acid Base Reaction Chegg Com
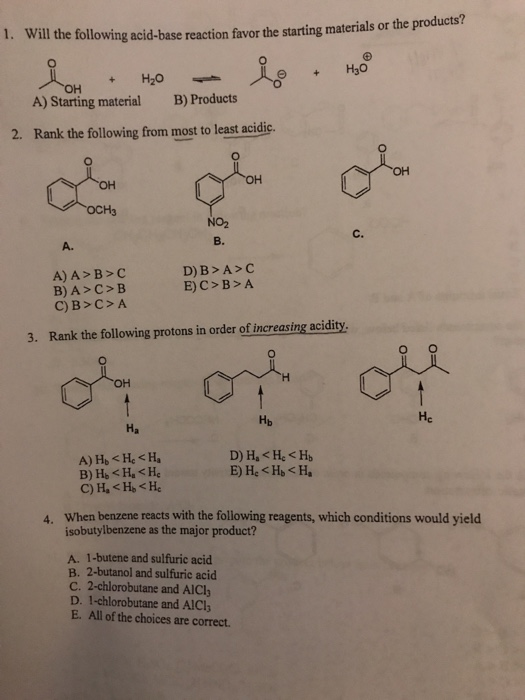
Solved Will The Following Acid Base Reaction Favor The Chegg Com
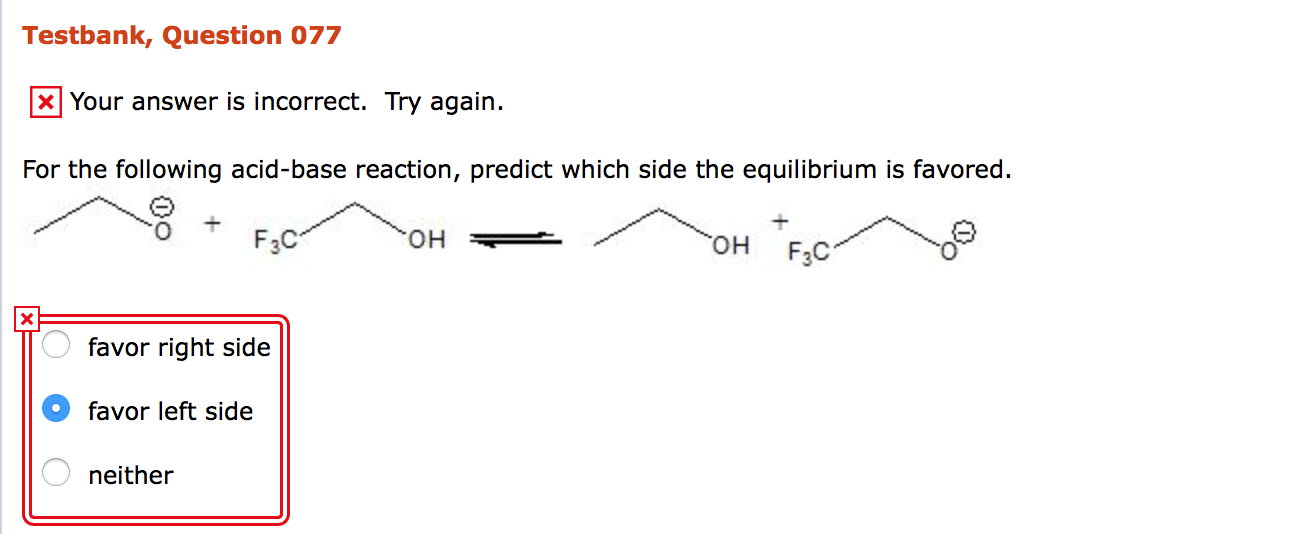
Solved For The Following Acid Base Reaction Predict Which Chegg Com

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps
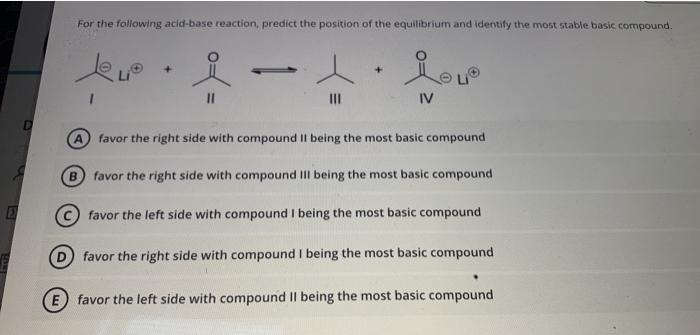
Solved For The Following Acid Base Reaction Predict The Chegg Com

Solved Which Side Will The Following Acid Base Reaction Chegg Com
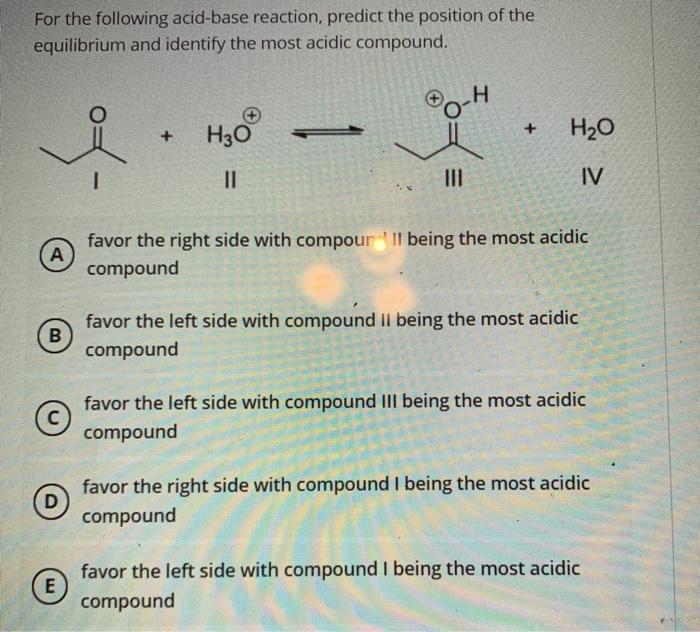
Solved For The Following Acid Base Reaction Predict The Chegg Com

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps
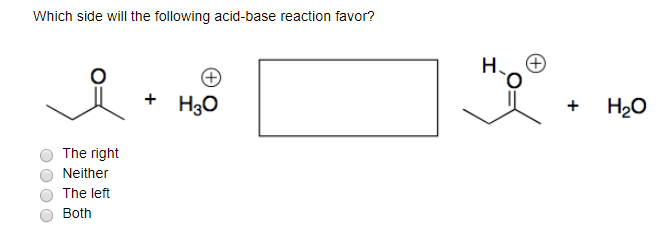
Solved Which Side Will The Following Acid Base Reaction Chegg Com

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps

How To Determine The Position Of Equilibrium For An Acid Base Reaction Chemistry Steps
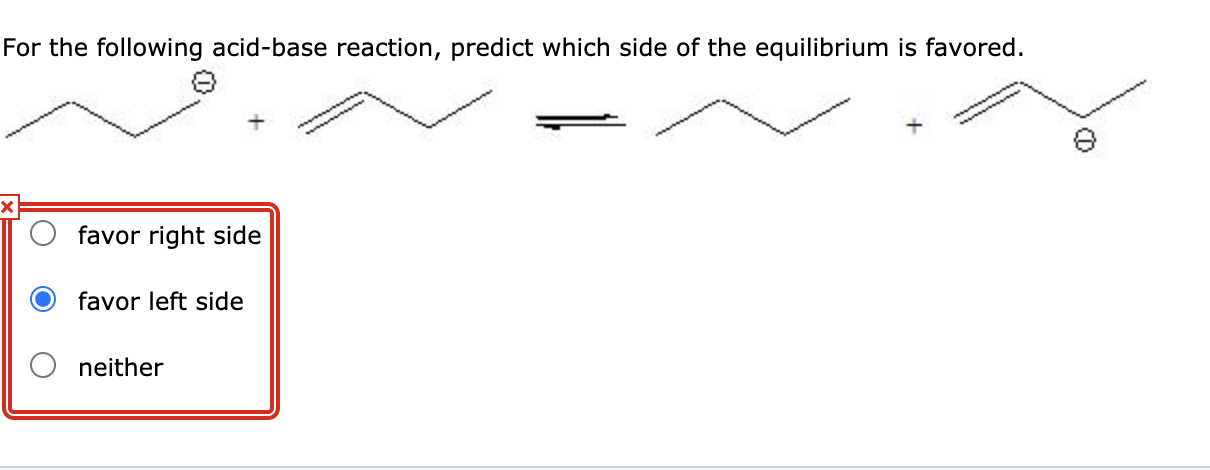
Solved For The Following Acid Base Reaction Predict Which Chegg Com

Which Side Of An Acid Base Reaction Is Favored Organic Chemistry Part 1 Youtube
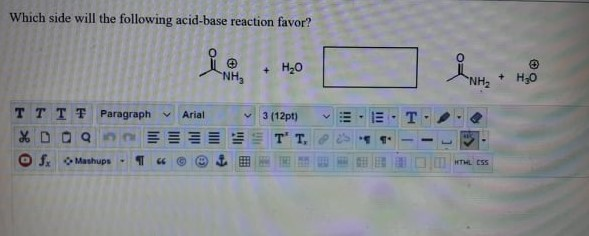
Solved Which Side Will The Following Acid Base Reaction Chegg Com
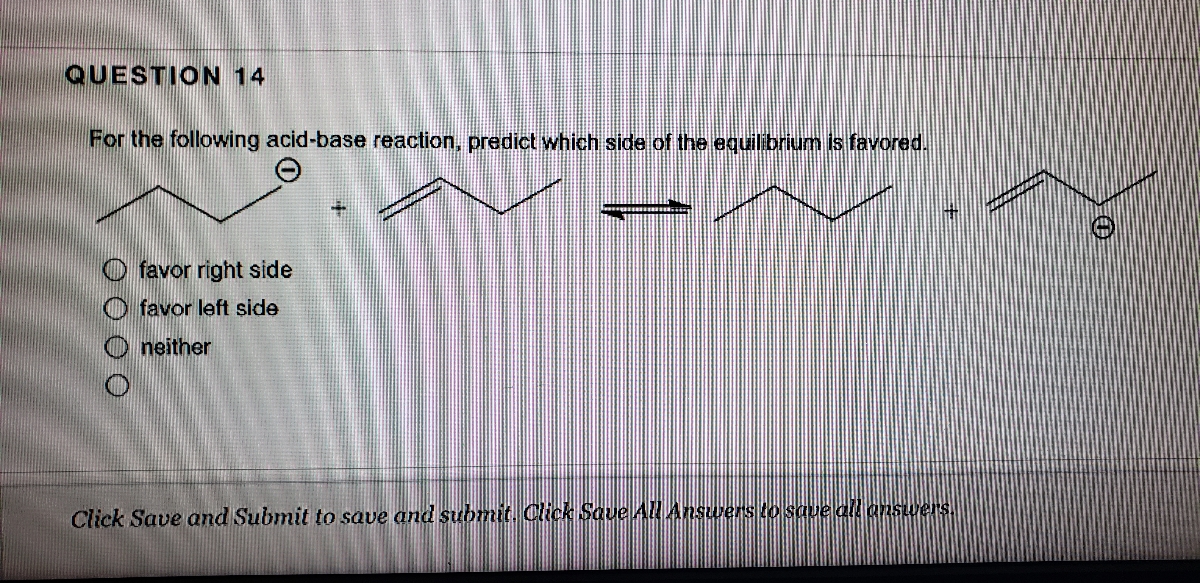
Answered Question 14 For The Following Acid Base Bartleby
Solved 1 Which Side Will The Following Acid Base Reaction Chegg Com
Comments
Post a Comment